In 2021, the Irregular Warfare Technical Support Directorate (IWTSD), on behalf of the Department of Defense, sought a field operator-friendly solution for the rapid detection of fentanyl. At that time, synthetic opioid detection relied upon the use of either external laboratory equipment or field detection kits with multiple reagents and buffers. Sample collection required the handling of the suspected opioid, increasing the risk of potential operator exposure. Field operators needed an easy-to-use detection system that could be used while wearing PPE and returned fast results in time-critical operational environments to ensure the safety of others.

With funding provided by the U.S. Navy, Signature Science chemists and engineers answered the need by developing a self-contained, single-use lateral flow immunoassay device prototype for the detection of fentanyl pharmaceutical-based agents. The prototype was demonstrated at the Defense Threat Reduction Agency’s Chemical Biological Operational Analysis events in 2022 and 2023. With valuable end user feedback and internal investment, Signature Science has optimized the prototype device and now offers the capability in a commercial product: FentAlert™. FentAlert™ can be operated in all levels of PPE and provides a presumptive field test indicating a clear positive or negative response for the presence of fentanyl in under 3 minutes.
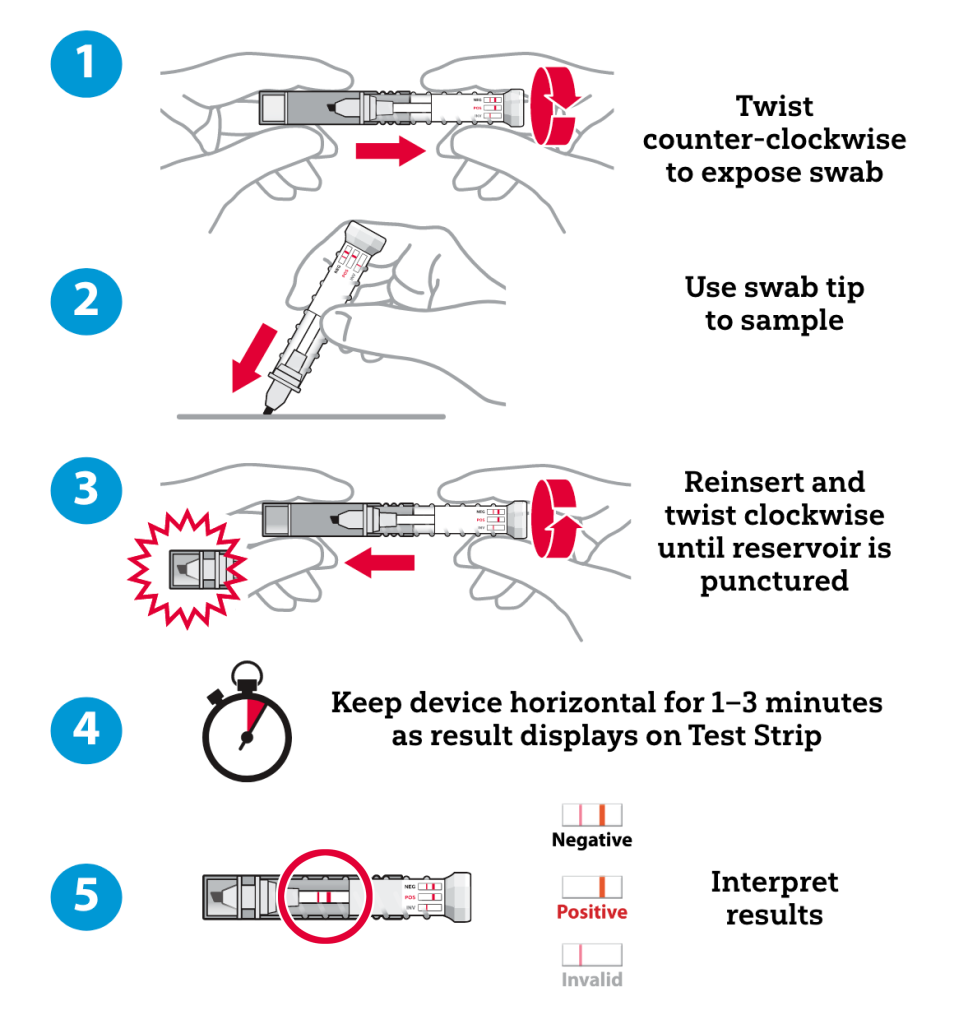
How FentAlert™ Works
Physical Features
| Dimensions | 13 cm tube |
| Weight | 10.76 grams |
| Operability | Fully operable in all levels of PPE |
Sampling & Analysis
| Sample Introduction | Sample with chisel tip swab; Analyze in sealed reservoir |
| Sample Type | Solid, liquid |
| Analysis Time | Positive or Negative results in 1–3 minutes |
Want more information about FentAlert™?
NOTE: This test (assay) provides only a preliminary qualitative result. Use a certified laboratory to confirm your results. Do not use this test for any products for human consumption. FentAlert™ is not FDA approved nor is it to be used to test human specimens. No clinical claims are made that FentAlert™ can mitigate or prevent disease. FentAlert™ is not to be used as a Drugs of Abuse Tests for human samples. The product is intended for use only by Governmental agencies engaged in field operations to determine whether a suspect material represents a hazard. FentAlert™ may only be possessed and used in accordance with state laws, as applicable.

