
Director, Quality Assurance & Data Science
Contact Molly
The National Guard Bureau (NGB’s) Weapons of Mass Destruction-Civil Support Teams (WMD-CSTs) are 57 specialized, active duty, full-time Guard units operating under federally approved CBRNE response doctrine and are individually certified by the Secretary of Defense.
Each WMD-CST fields a fully equipped mobile analytical laboratory system (ALS) in addition to sophisticated satellite communications systems; command and control systems; and chemical, biological, and radiological survey and monitoring equipment.
Fully-equipped Mobile Analytical Laboratory System (ALS)


The WMD-CSTs must operate in an environment that is geared toward providing rapid, accurate answers to life and death questions in the most chaotic environments imaginable.
In 2006, the NGB launched an initiative and contracted with Signature Science to pursue ISO 17025 accreditation for the WMD-CST laboratories. The objective was to standardize analytical tactics, techniques, and procedures and enforce scientific rigor across all WMD-CST ALS operations, ensuring that the ALS would always be ready to support the WMD-CST mission. Signature Science worked with NGB to develop and implement a quality program that achieved the goal of ISO 17025 accreditation for the entire network of 57 WMD-CSTs.
In 2006, Signature Science began working with NGB to develop a quality program to enable and support their goal of ISO 17025 accreditation for the entire network of 57 WMD-CSTs.
Building a Quality Program: Standardization
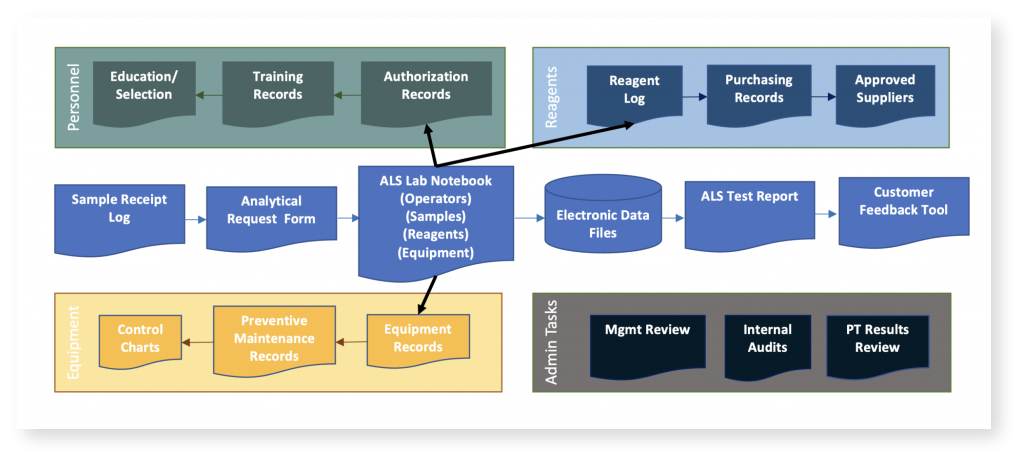
A key feature that supports achieving and maintaining accreditation is the analytical proficiency testing (PT) program Signature Science developed specifically for the WMD-CSTs and tailored to their mission. Unlike a commercial laboratory that analyzes hundreds of samples a day using standardized methods for method-specific target analyte lists, WMD-CST ALS operators might analyze only a handful or fewer samples for a given response action, but the variety of sample types and the possible targets are almost unlimited.
The WMD-CST PT program, developed and conducted under Signature Science’s ISO 17043 PT provider accreditation, encompasses the full suite of ALS analytical capabilities. WMD-CST PTs cover chemical, biological, and radiological analytical methods in a variety of environmental matrices. The tailored program includes approximately one PT round per month and all ALS operators participate in each round to practice their analytical skills, gain confidence in their analytical abilities, and identify potential training needs.
In addition to ensuring that operators can achieve the correct result for the samples provided in the PT, Signature Science’s approach to the WMD-CST’s PT assessment process also includes an in-depth skills evaluation that helps operators understand why they may have missed components of interest in a test sample or had other technical or quality issues. The program also offers insight into strengths and weaknesses at the WMD-CST unit and Program levels.
Signature Science routinely monitors available performance data for trends and issues that are reported to NGB and visits WMD-CST sites each year to assist in implementing best practices, correcting issues, and performing corrective action where needed, and preparing for accreditation assessments.
Biennial audits are performed for all 57 CSTs through NGB’s Standardization Evaluation and Assistance Team (SEAT) Program. Signature Science assists the WMD-CSTs in preparing corrective action reports (CARs) in response to SEAT visits, PT failures, and ISO 17025 audit deficiencies, and submits CARs to the ISO 17025 accrediting body on behalf of the WMD-CSTs.
Signature Science established document control procedures and supports NGB by drafting, reviewing, and revising Program documents to ensure ongoing ISO 17025 compliance, continuous improvement, and defensibility of results.
The quality management system is continually updated to ensure it remains effective and compliant with ISO 17025. When the ISO 17025 standard was updated in 2017, Signature Science was able to efficiently revise all program documents and procedures to meet the requirements of the new version of the standard (ISO 17025:2017), train the teams on the revised procedures, and ensure there were no gaps in accreditation for any of the WMD-CSTs. In addition, beginning in spring of 2019, NGB started fielding new mobile laboratories to the WMD-CSTs, including new instrumentation. Signature Science updated the quality management system to ensure teams would maintain their accreditation through this upgrade process, identified areas for improvement with the new analytical methods and equipment, and modified the PT program to ensure samples would be compatible to both analytical platforms throughout the fielding process. Ongoing maintenance and improvement to the quality system developed for the WMD-CSTs has proven critical to program success.
ISO/IEC 17025:2017 Transition
Want more information about laboratory quality assurance?
